Of the Following Molecules Which Has the Largest Dipole Moment
Hcl as the largest Ionic character because as we move up the group electro-negativity increases. C HF molecules have a smaller dipole moment.

Which Of The Following Has The Highest Dipole Moment Youtube
CS2 SeS2 PCl3 P is the central atom Who are the experts.

. D HF is much less soluble in water. NH3 BF3 NF3. This problem has been solved.
Is CCl4 dipole dipole. Which of the following benzene-like molecules do you expect to have the largest dipole moment. As discussed above in CCl4 C-CL has some value of dipole moment and is polar in nature but overall CCl4 molecule is nonpolar in nature because the net dipole moment of CCl4 molecule is zero.
HF has largest dipole moment because electronegativity difference of both is high so it is highly polar. NF3 BF3 NH3. Justify the statement based on the shapes of CO2 and H2O.
HCl 108 HBr 080 HI 042. The relatively high boiling point of HF can be correctly explained by which of the following. Which molecule has the largest dipole moment.
CH3Cl has larger dipole moment than CH3F because dipole moment is based on the product of distance and charge and not just charge alone. Of the following molecules which has the largest dipole moment. Solve any question of Chemical Bonding and Molecular Structure with- Patterns of problems.
Which of the following molecules have net dipole moments. Hence they are non-polar. Which of the following statements regarding resonance structures is total garbage.
Best answer Answer is. The Dipole Moment is defined by the charge separated multiplied by the distance separation between the charges. Which of the following molecules has the largest dipole moment net dipole.
Sp2 The hybridization of Br in BrF3 is dsp3 or sp3d The hybridization of Cl in ClF2 is sp3 A π pi bond is the result of the. SO2 The hybridization of the central atom in O3 is. Dipole moment is used to predict the shape of molecules.
2 on a question Of the following molecules which has the largest dipole moment. None of these D. The molecular structure of SOCl2 is pyramidal Which of the following molecules has a dipole moment.
Which of the following molecules would exhibit the largest dipole moment. Which of the following molecules has the largest dipole moment. Of the following molecules which has the largest dipole moment.
These are the actual values. BF3 NF3 NH3. HF has the largest dipole moment you can tell which molecule has the largest by looking on the periodic table they are usually the pair that are furthest from each other and it is also due to them having the biggest difference in electronegativity usually the closer two elements are the weaker the dipole moment.
D CCl4 A molecule which has a symmetrical geometry will have no dipole moment as the magnitude of all the bond moments cancel each other. These molecules have tetrahedral geometry due to sp3 hybridization of carbon atoms. Experts are tested by Chegg as specialists in their subject area.
A CC B CH C CO D HN E HF Ans. Molecular polarity molecular dipole bond polarity Section. Which of the following covalent bonds has the largest dipole moment.
Prev Question Next Question. Select all that apply. Of the following molecules the one which has permaanent dipole moment is.
The structure of compounds given in options are as follows Thus CCl4 has no dipole moment. B HF is the strongest acid. Question Which molecule has the largest dipole moment.
Which one of the following arrangements of molecules is correct on the basis of their dipole moments. Fluorine is more electronegative than chlorine but the carbon-fluorine bond is also much shorter than. A HCl B Hl C HBr D HF Medium Solution Verified by Toppr Correct option is D HF has largest dipole moment because electronegativity difference of both is high so it is highly polar.
We know that for symmetrical compounds the dipole moment is always zero. Therefore BF₃ CO₂ and CF₄ are all non-polar molecules and NCl₃ has the largest dipole moment Which has the largest dipole. A HF gas is more ideal.
Among the following molecules the one. E HF molecules tend to form hydrogen bonds. Hence B is the right answer.
Click to see full answer. 111 15 Difficulty Level. WELL to figure out dipole moments you need to know a bit abt theelectronegativity of the elements so tht you can approximate thecharge on each View the full answer Transcribed image text.
Hence C H 2 C l 2 has the highest dipole moment amongst the given options. The liquefied hydrogen halides have the normal boiling points given above. HF has the largest dipole moment you can tell which molecule has the largest by looking on the periodic table they are usually the pair that are furthest from each other and it is also due to them having the biggest difference in electronegativity usually the.
O CHCl3 O CH4 O CH2Cl2 CH3CI. HF has the largest dipole moment you can tell which molecule has the largest by looking on the periodic table they are usually the pair that are furthest from each other and it is also due to them having the biggest difference in electronegativity usually the closer two elements are the weaker the dipole moment. All resonance structures must have the same number of electrons All resonance structures must differ in the hybridization of.
Select all that apply. We review their content and use your feedback to keep the quality high.
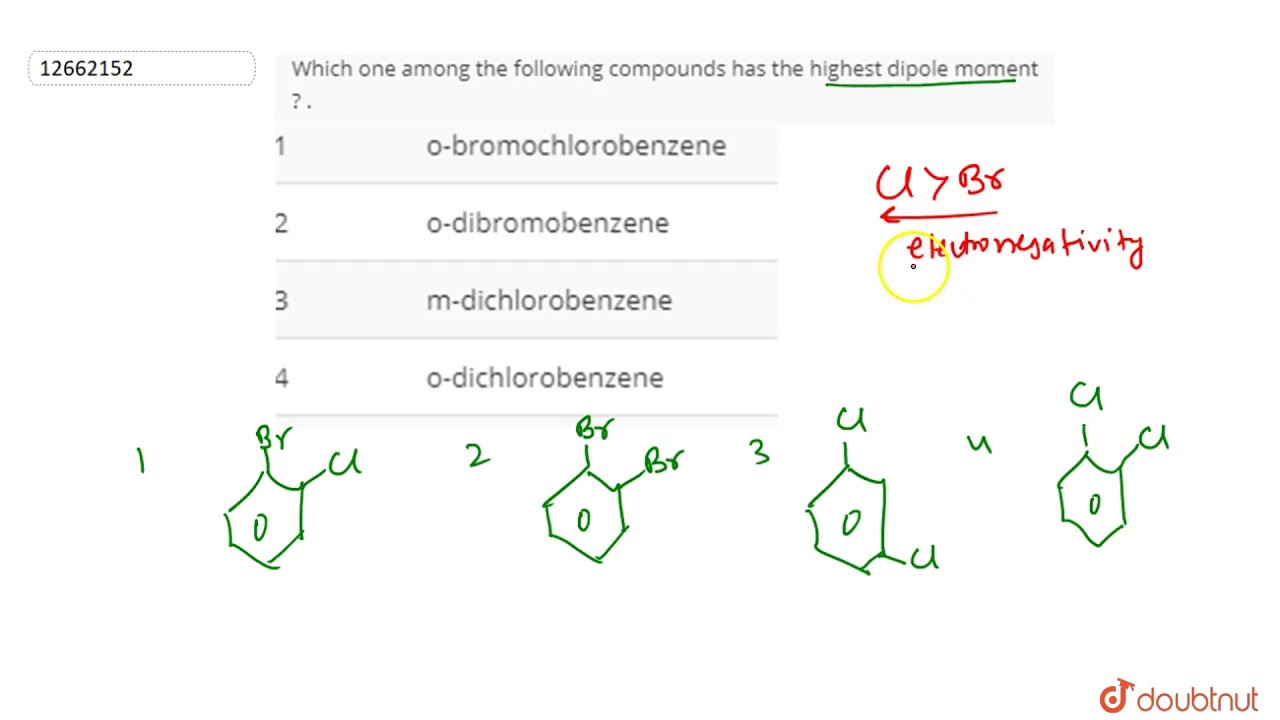
Which One Among The Following Compounds Has The Highest Dipole Moment Youtube

Which One Of The Following Molecules Has The Largest Dipole Moment Electro Negativity Values Of Elements Concerned Are B 2 0 Cl 3 0 F 4 0 H 2 1 N 3 0 O 3 5 P 2 1 S 2 5

Solved Which Of The Following Has The Largest Dipole Moment A Mathrm Hcn B Mathrm H 2 Mathrm O C Mathrm Ccl 4 D Mathrm So 2
No comments for "Of the Following Molecules Which Has the Largest Dipole Moment"
Post a Comment